Enartis News – Reduction: how to prevent and treat it
Reduction is one of the most common problems in winemaking. Hydrogen sulfide and other volatile sulfurcontaining compounds (VSC) are generally produced during alcoholic fermentation but can also develop during storage, ageing and post-bottling. The aromas generated by these sulfur compounds are described as rotten egg, burnt rubber, skunky, burnt match, asparagus, onion and garlic. Additionally, they can impact mouthfeel and intensify other negative wine attributes such as bitterness and herbaceous characters. Their presence, when close to or above the sensory threshold, decreases wine aromatic quality. For this reason, it is important to know how to prevent and treat this defect during the different stages of winemaking.
ORIGIN OF REDUCTION
Alcoholic fermentation: the beginning of reduction
The first mechanism is related to the synthesis of amino acids. Yeast produce hydrogen sulfide (H2S) as a normal step in the synthesis of sulfur-containing amino acids. This explains why accumulation of H2S often occurs in cases of nutrient deficiencies associated with amino acid production, such as low assimilable nitrogen or vitamin deficiencies, which are important cofactors in the synthesis of methionine. Genetic differences associated with amino acid production explain why some yeast strains are reported to be low or high H2S producers. A second mechanism is the transformation of elemental sulfur to H2 S. Elemental sulfur is commonly sprayed in vineyards to control powdery mildew. Formation of H2S from S-residues cannot be corrected by nutrient addition and is much less dependent on yeast genetics. H2S is usually formed from S-residues during the second half of fermentation when carbon dioxide stripping effect is weaker. As a result, H2S persists in the wine.
SO2 management at the end of alcoholic fermentation: an underestimated critical point
An often overlooked cause of H2 S formation is the early addition of sulfur dioxide at the end of alcoholic fermentation. The enzymatic activities of fermenting yeast remain active for at least 10-15 days after the end of alcoholic fermentation. An addition of SO2 during this phase activates the sulfite reductase pathway, a protective mechanism for yeast to transform this toxic compound into the more harmless H2 S.
This is the reason why, in presence of lees, it is recommended to wait at least two weeks before proceeding with adding sulfites.
EnartisStab MICRO M (antimicrobial preparation of specially activated chitosan designed for the treatment of cloudy wine) is an effective alternative to the early addition of SO2 for protecting wine from spoilage micro-organisms. EnartisTan SLI (ellagic tannin from untoasted American oak) can replace the antioxidant effect of SO2 .
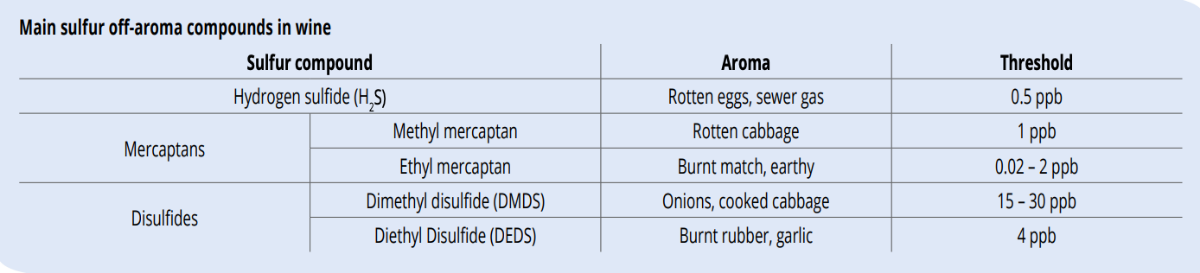
COMPOUNDS RESPONSIBLE FOR SULFUR OFF-AROMAS
Hydrogen sulfide (H2 S)
Among the sulfur compounds, H2S is the most common and infamous sulfur off-aroma. H2S has a low sensory threshold and an odor reminiscent of rotten eggs. While appropriate nutrition management is the best way to prevent excessive H2S formation during fermentation, there are several approaches to remediating H2S:
- H2S is highly volatile and can be readily removed through sparging with inert gas..
- H2S is easily oxidized, so aeration may also be used. However, oxidation can cause a loss of desirable S-containing compounds if done in excess, such as thiols critical to Sauvignon Blanc for example.
- Copper addition is a common practice for the removal of H2S since the complexation of copper with this compound causes it to precipitate.
If H2S is not removed quickly, it can result in the formation of more problematic sulfur containing compounds, mercaptans.
Mercaptans
This is a large group of very pungent sulfur compounds among which ethyl and methyl mercaptan are the most well-known. In the presence of methyl and ethyl mercaptan, aeration should not be attempted. Mercaptans are readily oxidized to form other lesspotent compounds, e.g. to their corresponding disulfides, which are significantly harder to remove. Mercaptans can be removed to some extent with appropriate copper additions, though this operation has been found to be only about half as efficient as H2S removal. The reaction should form an insoluble copper salt that can be filtered from wine (see further).
Disulfides
Mercaptans can oxidize to form disulfides when exposed to oxygen. These new compounds smell like garlic, canned asparagus, burnt rubber and onion and are almost impossible to eliminate. The chemical change induced by the oxidation from mercaptan to disulfides increases their sensory threshold and changes their ability to bind to copper. Therefore, while mercaptans react with copper, their oxidized form cannot react. Disulfides can be reduced back to mercaptans, then can be removed by copper. This is the main concept of using ascorbic acid in combination with copper sulfate or copper citrate as a treatment. Disulfides are first reduced with the addition of 50 mg/L or more of ascorbic acid, immediately followed by an appropriate addition of copper. This reaction can take a couple of months and it is important that free SO2 levels are adequate before adding ascorbic acid, which can increase the potential for wine oxidation.
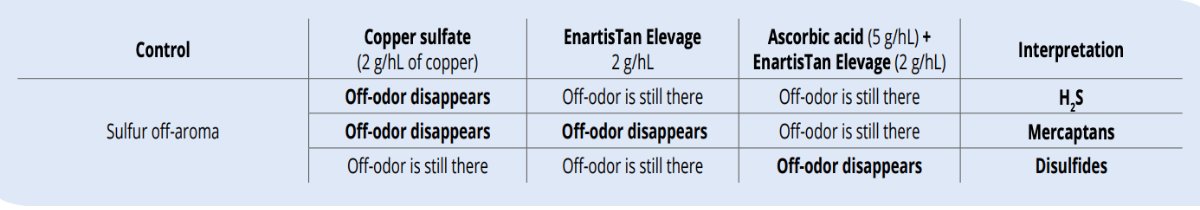
How to recognize the cause of sulfur off-aromas
The best way to assess the problem is sensory screening before deciding on further treatment. A simple trial consists of taking a wine with sulfur off-aroma, pouring it into 4 glasses where one glass is the control, copper sulfate is added to the second glass, EnartisTan Elevage is added to the third glass and the fourth glass is treated with ascorbic acid and EnartisTan Elevage. Interpretation of the results is given in the table here below.
WHAT TREATMENTS ARE AVAILABLE?
Aeration
Aeration can contribute to the volatilization of H2S. Furthermore, exposure to oxygen will lead to the transformation of low sensory threshold mercaptans to less pungent disulfides. This might initially appear to improve organoleptic qualities, but as mentioned before, disulfides can be hard to remove and still impart off aromas. To avoid oxidation of these sulfur compounds when attempting to remove H2S with aeration, use an inert gas like nitrogen and be aware of the volatilization of other positive volatile aromas.
Copper
Copper is commonly used in the treatment of reductive characters. It reacts with H2 S and certain mercaptans but does not react with disulfides. Furthermore, these reactions may require the addition of copper in excess, which can also affect fruity volatile thiols, causing a decrease in aromatic complexity. The other issue with excess copper is its ability to catalyze oxidation reactions, leading to premature ageing, as well as the formation of cupric haze.
Recent studies have shown that, contrary to conventional wisdom, copper-sulfide complexes are not readily removed by racking and can even pass through some types of filtration. Moreover, these complexes can recycle bound sulfur compounds over time, revealing sulfur off-aromas post bottling.
In order to minimize the risk of residual copper, it is recommended to use a fining mixture containing copper like Revelarom as a corrective and preventive for sulfur aromas. The specific combination of organic and inorganic fining agents present in its formulation helps to effectively remove the copper-mercaptan complex and prevent residual copper accumulation in finished wines.
In the event of high residual copper, there are several options for removal. Among them:
Bentonite fining (PLUXCOMPACT) and yeast hulls (SURLÌ ONE) can help remove small amounts of copper, between 0.1-0.2 mg/L.
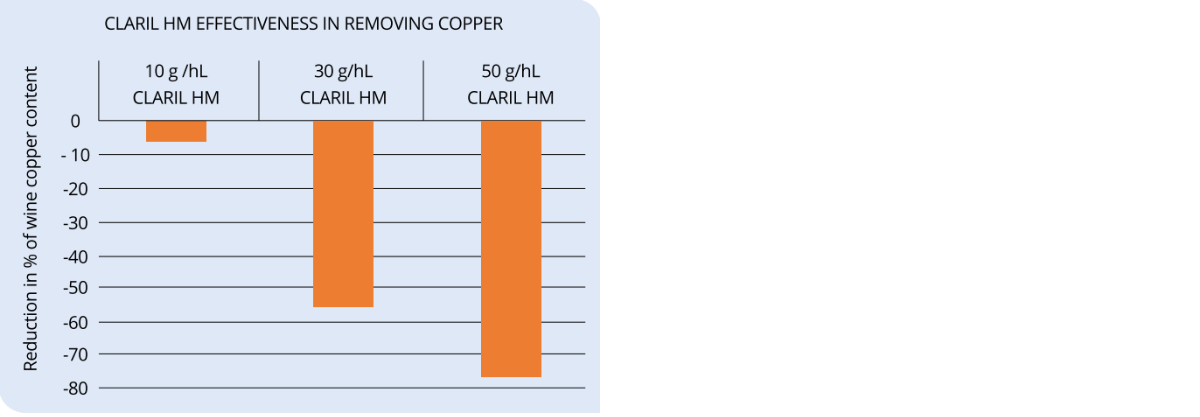
CLARIL HM is a product made of co-polymer of polyvinylimidazole and polyvinylpyrrolidone (PVI/ PVP) and chitosan. PVI/PVP is an adsorbent with high selectivity for metals. Its application in winemaking is mainly due to its ability to remove coper and iron. Chitosan reinforces PVI/PVP’s effect, especially towards copper.
Tannin Addition
The addition of tannins, especially ellagic and condensed tannins, can bind and react with mercaptans to form odorless complexes. These complexes are very stable over time and do not entail the post-bottling risk of sulfur off-aroma appearance. EnartisTan ELEVAGE (ellagic tannin obtained from light-toasted French oak), EnartisTan SLI (ellagic tannin from untoasted American oak) and EnartisTan CŒUR DE CHÊNE (ellagic tannin from toasted French oak) are very effective in scavenging mercaptans and can successfully replace the addition of copper prior to bottling. EnartisTan MAX NATURE (condensed tannin extracted from exotic species wood) is another option particularly recommended for treating easy-to-drink, light wines.