Enartis News – Tannins: nature, origin and enological effects
TANNINS IN NATURE
Tannins, just like antibodies in the animal world, have the role of protecting plants from external aggressions. For example, their bitter and astringent taste repel animals that try to eat these plants or their fruits. When a viral, bacterial or fungal infection is underway, tannins limit pathogen growth, oxidation and the deterioration of attacked tissue. To ensure protection from a large number of microorganisms, evolution has led to the development of tannins differing in composition and chemical structure. Typically, tannins are subdivided in two categories:
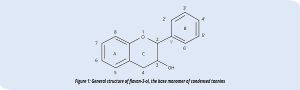
- Condensed tannins. Oligomers and polymers of flavan-3-ols (Figure 1), which are also known as proanthocyanidins because, by hot acid hydrolysis, they release an anthocyanidin from which their name is derived.
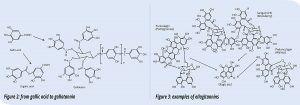
- Hydrolyzable tannins. These are composed of a sugar, usually glucose, esterified with gallic or ellagic acids that are named gallotannins (Figure 2) or ellagitannins (Figure 3), respectively.
The abundance of different forms of tannins allows them to bind to various proteins, and hence express their antimicrobial action efficiently against different pathogen species. When a microorganism attacks plant tissue, tannins can alter the activity of transport proteins in the cell membrane as well as enzymatic proteins and consequently limit pathogen proliferation.
Tannins can also chelate metals. Metals such as copper, iron, aluminum, zinc, magnesium and manganese are essential cofactors for the proper function of many enzymes; therefore the absence of these metals has a negative effect on microorganism growth. Furthermore, metals such as copper and iron are involved in oxidative processes that lead to the formation of free radicals in the presence of oxygen. Due to their chelating capacity, tannins also have an antioxidant effect.
TANNINS IN WINEMAKING
The generic term, winemaking tannin, is used to indicate a variety of plant extracts that have similar characteristics; they have a bitter taste and precipitate proteins. However, as we will see, both the tannin origin and extraction technique significantly affect the winemaking properties of the tannin.
In fact, there is a large range of products available. Among gallotannins, those most commonly used in winemaking are gallnut tannins, extracted from galls of different species that range from Aleppo oak (Quercus infectoria) to Rhus semialata, from which a well-known Chinese gall tannin is extracted. Tara
tree (Caesalpina spinosa) and Myrobalan (Terminalia chebula) fruit are also good sources of gall tannins, as are sumac leaves (Thyphina spp.).
Ellagitannins are found in chestnut (Castanea sativa) or oak wood (Quercus robur or Q. petrae). Whereas condensed tannins can be extracted from grape seeds and skins or from mimosa bark, but more often they are produced from quebracho wood (Quebrachia spp.)
Winemaking tannin production
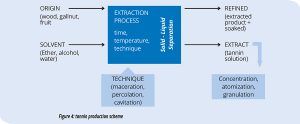
As shown on Figure 4, the tannin production process is relatively easy. To obtain a high-quality product it is important to select extraction and drying techniques that do not alter the original structure of the tannin. The extraction solvent choice, extraction and drying duration and temperature, and protection from oxygen are all factors that determine the total polyphenol content as well as the amount of tannin extracted in its native form.
Effects of tannins in winemaking
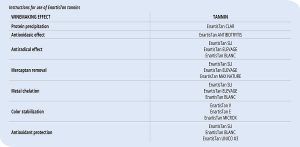
Tannins used in winemaking have various known proprieties, many of which have been known for a long time and some discovered or observed in daily winemaking practices that now, thanks to research equipment, the mechanisms at play can be studied and analyzed. In general, we can say that all tannins have all the proprieties attributed to this compound class. However, in some cases, it is possible to identify a certain specific action linked to the tannin composition and chemical structure (Table 1).
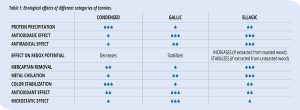
Protein precipitation
The capacity to react with proteins, resulting in their precipitation, is the tannin property that has been known the longest and lead to their initial use in winemaking. Linked with this property is also its astringent character, typical of tannins. The reactivity with regards to proteins varies according to the nature of the tannin. Proanthocyanidins are usually more effective than ellagitannins and gallotannins in overfined wines or wines rich in endogenous proteins.
Even so, several gallotannins and ellagitannins have the ability to remove proteins.
Antioxidasic effect
The presence of laccase produced by Botrytis cinerea in must obtained from mold affected grapes causes uncontrollable oxidation of extracted phenolic compounds which continues in the wine. The addition of exogenous tannins to must, particularly hydrolyzable tannins, can limit the action of these feared oxidases. Gallotannins, due to their structure and size, can disrupt the active site of the enzyme.
Ellagitannins, on the other hand, can chelated copper, an indispensable cofactor for laccase activity.
Antiradical effect
Phenolic substances are known for their capacity to capture free radicals. This property is of great medical interest because live tissue is protected from the degenerative action of free radicals. For this reason, tannins are used in the production of anti-aging supplements and are considered the origin of the French Paradox. Due to the rich presence of OH groups and the characteristic links between benzene rings, ellagitannins are the most efficient.
Effect on redox potential
Tannins are electroactive compounds. When added to wine without oxygen, they have a notable effect on the redox potential of white wines; the effect is lower in red wines because the phenolic content acts as buffer. In general, it is possible to observe different behaviors based on the nature of the tannin. Ellagitannins extracted from toasted wood increase the redox potential; gallotannins and ellagitannins extracted from untoasted wood have a minimal effect, whereas proanthocyanidins decrease redox potential.
Mercaptan removal
Mercaptans are sulfur compounds that can develop from hydrogen sulfide produced during alcoholic fermentation of unclean or excessively sulfited must. These compounds are responsible for odors such as cabbage and onion which, when found in concentrations above the sensory threshold, decrease perceived wine quality. Tannins, particularly ellagitannins, can reduce mercaptan content in a hydroalcoholic medium. There seems to be two mechanisms at the origin of this phenomenon: on one hand, a direct condensation reaction between tannins and mercaptans; on the other hand, a coupled oxidation that brings about the formation of hydroperoxides and free radicals capable of destroying mercaptans.
Metal chelation
One of the tests used to identify an enological tannin is, that when placed in a water-based solution of ferric salts, it reacts with the metal to yield a dark precipitate. Tannins, indeed, combine with metals in cationic form to produce chelates, some of which will precipitate. Ellagitannins and gallotannins have a greater reaction capacity than proanthocyanidins. Obviously, the chelation effect is a decrease in the copper and iron content, which even when minimal, reduces the sensitivity of wine to oxidation.
Microbiostatic effect
For some time, tannins have been known for their bacteriostatic effect. In winemaking textbook from the beginning of 20th the century, tannins were already described as “antiseptic” in the prevention of the “ropy” fault (Pediococcus spp.) and tannin use was highly recommended for the treatment of grapes that were not completely healthy in order to avoid the proliferation of undesirable bacteria. In fact, some laboratory experiments demonstrated the delay of Oenococcus oeni malolactic bacteria development in wines that had been treated with tannins of different nature, particularly gallotannins. As already mentioned, the microbiostatic action can be justified by the mechanism of interaction between tannins and transport proteins present in the microorganism cell walls.
Color stabilization
Tannins are among the primary actors regarding color stability in red wine. This effect is due to their capacity to consume oxygen which, in turn, prevents anthocyanin oxidation, as well as their capacity to combine directly with anthocyanin fractions to yield pigments that are less sensitive to oxidation or to form stable pigments. Gallotannins mainly have an indirect action in the protection of anthocyanins from oxidation; ellagitannins favor the oxidation of ethanol to acetaldehyde, hence increasing the formation of bonds between condensed tannins and anthocyanins via acetaldehyde bridges. Condensed tannins, and particularly those with two OH groups in meta position in the A ring, are the only ones capable of reacting directly with anthocyanins to form stable compounds.
Sensory effects
In a hydroalcoholic solution, tannins are bitter and astringent. The intensity of these sensations varies greatly depending on the tannin origin and composition. Chestnut and gallnut tannins are more bitter than oak or quebracho tannins. This bitterness is due to the presence of digallic acid and coumarin heteroside; chestnut and gall tannins have quantities that are higher than the perception threshold.
Astringency is a characteristic that is linked to the reactivity of the tannin with saliva proteins. Oak tannins also have a characteristic woody aroma which is particularly accentuated when the wood from which they have been extracted from has been toasted. The mouthfeel of the tannin varies according to the dosage and type, but it is also dependent on the physical and chemical characteristics of the wine. For this reason, every tannin addition should first be tested on a small scale to determine the correct dosage.