Enartis News – Wine tartrate stability
The presence of tartrate crystals in a bottle is commonly perceived as a fault by consumers. Crystals are mostly composed of potassium bitartrate but precipitation of calcium tartrate is becoming more and more frequent. While the formation of potassium salts can be prevented with the use of protective colloids, calcium stabilization requires specific interventions.
POTASSIUM BITARTRATE
Testing wine potassium tartrate stability
The Minicontact test (measurement of wine conductivity variation before and after refrigeration for 30 minutes with potassium bitartrate seeding) and the cold test (6 days storage at -4°C) are quite reliable tests for evaluating wine stability.
Wineries that do not have access to the Minicontact test and that want to save time, often prefer to use the freeze/thaw test (wine is frozen for a few hours and then thawed before being inspected for any sign of crystalline tartrate precipitation). This method overestimates wine instability. As ice formation occurs, all solutes, including potassium, tartaric acid and alcohol, are concentrated and the precipitation that happens is not related to wine concentration condition. Furthermore, ice formation damages protective colloids structure that consequently lose their stabilizing effect. For this reason, its use is not recommended.
Potassium bitartrate stabilization
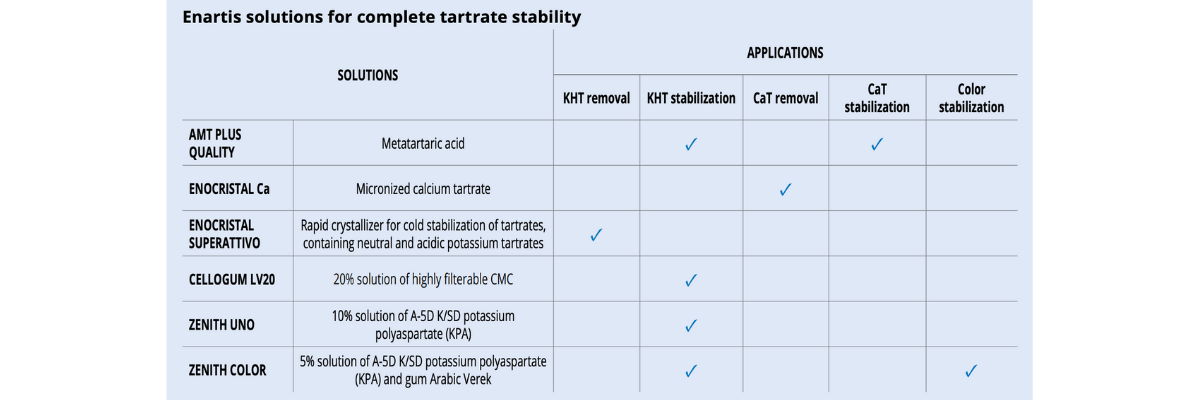
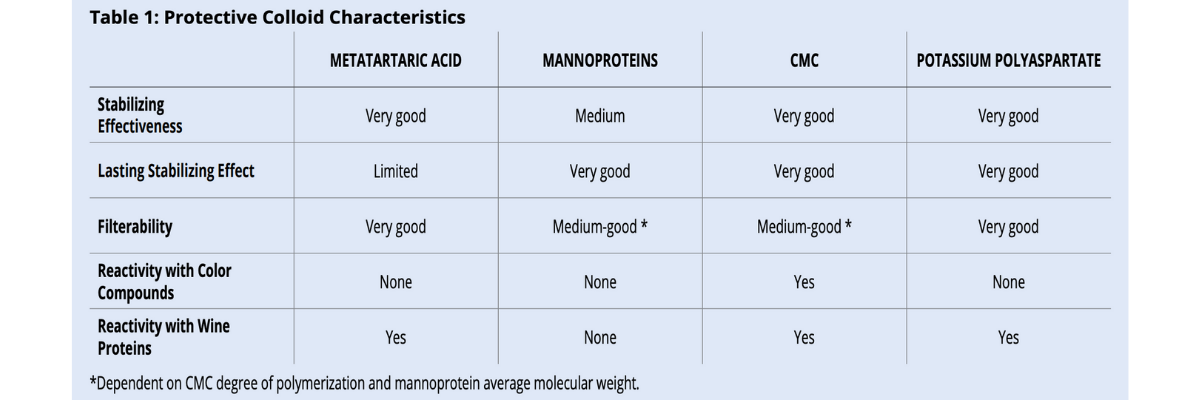
There are various methods that can be used duringthe winemaking process to reduce potassium bitartrate (KHT) precipitation in bottled wines. Some techniques are considered “subtractive” and involve reducing the concentration of tartaric acid and/or potassium in wine (tank cooling, electrodialysis, cation exchange resins). Other “additive” techniques make use of protective colloids or crystallization inhibitors which can be added to wine. Additive techniques are more respectful to sensory qualities and environmentally friendly. However, not all permitted protective colloids are effective in the same way and some of them have specific limits under certain circumstances (table 1).
Protective colloid stabilizing mechanism
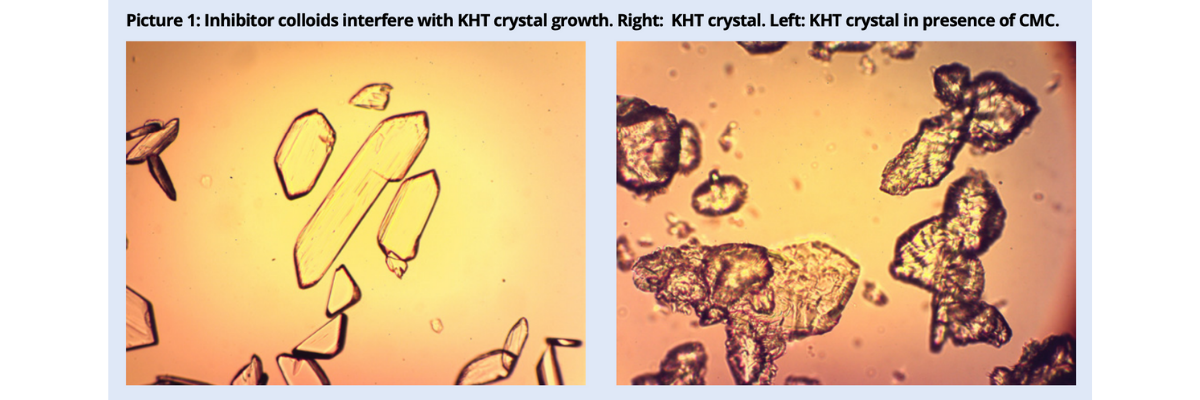
Despite the differences among protective colloids, the stabilizing effect depends on their capability of opposing the growth of the nuclei around which crystals are formed. If the dose is too low, inhibition is only partial and anomalies and unevenness are observed in the shape of the crystals (picture 1).
Wine suitability for stabilization with colloids
When protective colloids are used for KHT stabilization, wine must meet some requirements:
1) Protein stability
Metatartaric acid, CMC and potassium polyaspartate (KPA), unlike mannoproteins, are quite reactive with wine proteins due to their high negative charge. For this reason, it is imperative to check wine protein stability and be sure wine is well below the maximum stability limit, whatever the analytical method used. Wines close to the stability limit can form haziness or sediment whenmetatartaric acid, CMC and/or KPA are added. Preliminary lab addition trials can easily be performed and are very useful for preventing problems and extra labor in the cellar. If haziness appears, bentonite fining is necessary.
2) Color stability
Color stability is a necessary requirement for all red wines, regardless of whether they are tartrate stabilized through the use of colloids.
For CMC, it is important to note that it can cause color precipitation in color stable wines. For this reason, the use of CMC in red wines must be always combined to the addition of a gum Arabic effective for color stability. The new European Regulation (EU) N. 2019/934 is going to forbid the use of CMC in red and rosé wines starting from December 2019. All the other tartrate stabilizing colloids do not stabilize nor destabilize wine color.
3) Filterability
Wine filterability must be adequate for final filtration.
Metatartaric acid and KPA do not change wine filterability. Once they are homogenously distributed, wine can immediately be bottled.
On the contrary, mannoproteins and CMC can decrease wine filterability. CMC effect depends in its degree of polymerization (DP): the higher the DP, the greater the clogging effect. With high DP CMC, waiting 3-4 days between the addition and final microfiltration helps to bring the filterability index back to acceptable values. Mannoproteins can have very different average molecular weights depending on the method used for their production.
Consequently, they can have a different impact on wine filterability. Information provided by suppliers are helpful to understand how to manage filtration.
CALCIUM TARTRATE
Testing wine calcium tartrate stability
The main problem with calcium tartrate (CaT) is understanding if wine can form precipitate.
Stability tests used to verify KHT stability (cold test and minicontact test) do not confirm if wine is calcium unstable. The reason is that cold temperatures have little effect on the rate of CaT precipitation and the tests are not long enough. In fact, wine calcium content is very low compared to potassium and this is one of the reasons why the spontaneous formation of nuclei of crystallization can take very long, even years, making CaT precipitation unpredictable.
Wine calcium content is the most used parameter to classify a wine stable or unstable. For red wines, 60 mg/L is considered the safe maximum limit, for white wine the limit is 80 mg/L.
A more reliable test consists of seeding a sample of wine with micronized calcium tartrate and measuring the quantity of calcium precipitated after 2 days storage at a cold temperature. Calcium precipitation above 25 mg/L indicates a high risk of CaT instability.
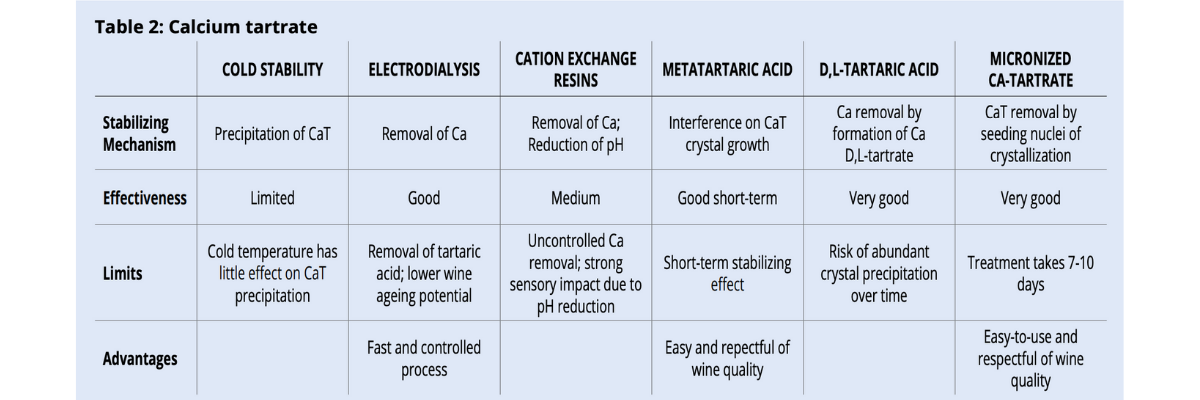
Calcium tartrate stabilization
The only colloid that has a significant effect for CaT stabilization is metatartaric acid. Unfortunately, since it hydrolyzes quickly, its effectiveness is short and can only be used for wine with a very rapid rotation.
Alternatively, techniques based on calcium or CaT removal provide a more reliable and long-lasting stability (table 2).
This can be achieved by using physical treatments or chemicals.
Electrodialysis is the most effective among the physical treatments, but this technology is not broadly available to everyone and, by removing tartaric acid and potassium, it has an impact on wine sensory qualities and ageing potential.
Cation exchange resins help reduce calcium content, but their effectiveness on CaT stability is mainly due to their action of lowering wine pH. Unfortunately, the lowering of pH is not always organoleptically pleasing.
Cold stability is not a reliable technique. Low temperatures do not significantly increase or accelerate CaT precipitation and the usual 7-14 days cooling treatment can be too short.
Calcium tartrate and D,L-tartaric acid are the only chemicals permitted by the OIV for CaT stabilization.
D,L-tartaric acid effectiveness is supposed to depend on its capability to form a calcium salt that is much more insoluble than the one formed by the L-tartaric acid naturally present in wine. In reality, this characteristic is not enough to make D,L-tartaric acid a reliable stabilizer. The formation of nuclei starting the crystallization process takes a long time and, because of the high insolubility of calcium D,L-tartrate, its application can easily cause abundant precipitation over time. Furthermore, D-tartaric acid is NOT generally recognized as safe, and neither is the racemic D,L mixture, because it can form kidney stones.
Calcium tartrate, and specifically micronized calcium tartrate, is used for seeding nuclei of crystallization. In this way, it is unnecessary to wait for the spontaneous and unpredictable formation of nuclei, while at the same time promoting a rapid formation and precipitation of crystals. Because of the insolubility of calcium tartrate, treatment can be done at cellar temperature, (10-15°C), avoiding cooling. Crystal precipitation takes approximately 7-10 days and guarantees wine stability without impacting wine sensory.
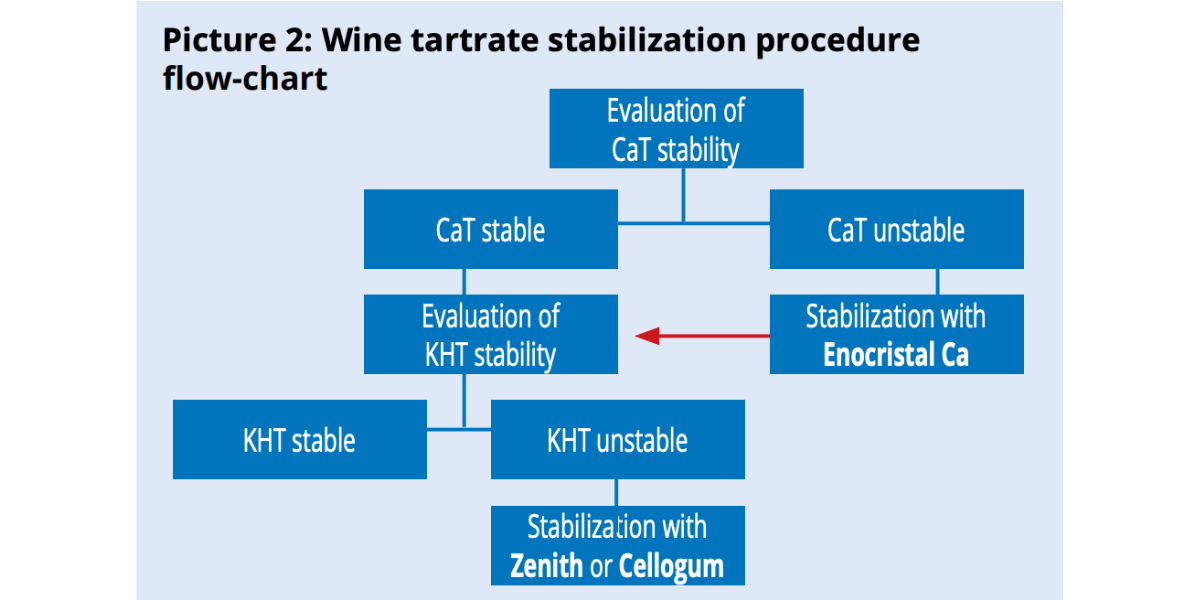
Since CaT precipitation induces KHT precipitation and not the other way around, it is more convenient to start stabilizing calcium first then potassium (picture 2).