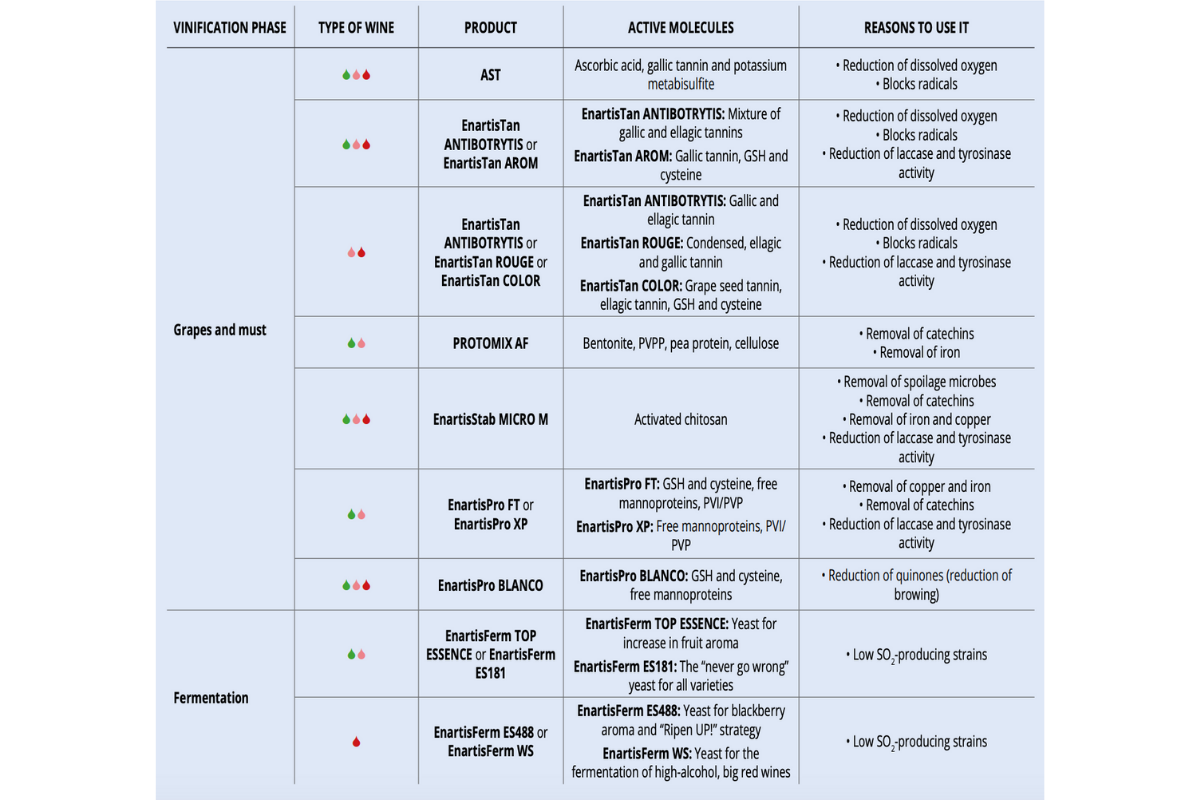
Enartis News – Replacing SO2 during fermentation
Market demand for low SO2 or SO2 -free wine is increasing as winemakers attempt to produce wines suitable for consumers suffering from food intolerances. For winemakers, embracing this philosophy means a greater commitment of time and responsibility in avoiding the risk of exposing an unprotected wine to chemical and microbiological modifications. With the recent approval of products such as chitosan and PVI/ PVP, it is now easier to replace sulfur dioxide.
SO2 ACTIVITIES
The three reasons for using SO2 in the pre-fermentation stage are to:
• Protect juice from oxidation
• Minimize laccase activity in the case of Botrytis infected grapes
• Control the growth of microorganisms that can damage wine quality.
ALTERNATIVES TO SO2 ANTIOXIDANT AND ANTILACCASE ACTIVITY
To better understand if and how SO2 can be replaced, it is necessary to know the mechanism behind the oxidation of juice and how SO2 can interfere with it.
Juice Oxidation Mechanism
Oxidation is considered one of the main problems encountered during vinification as it adversely affects the sensory properties of wine causing browning, loss of flavor and aroma, and increased astringency.
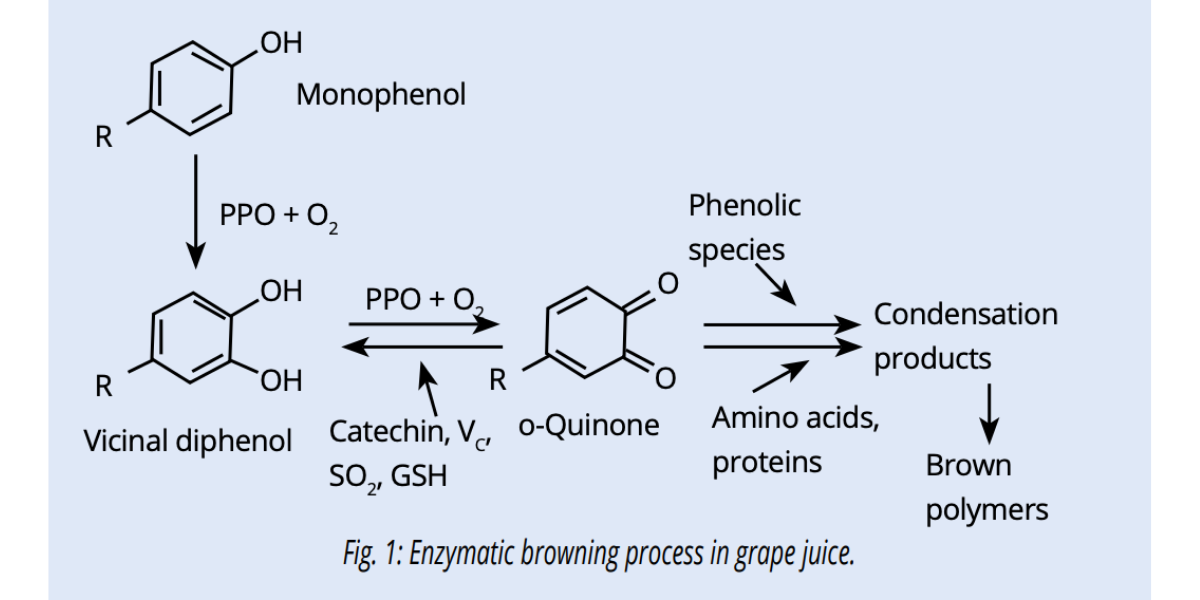
Juice oxidation can be classified into enzymatic (Fig.1) and non-enzymatic oxidation, with the former dominating on the latter. Many juice and wine compounds are susceptible to oxidation but phenolic compounds are most affected.
Enzymes responsible for phenolic compound oxidation at the juice stage are polyphenol oxidases (PPO):
tyrosinase in healthy grapes and laccase in Botrytis infected grapes. PPOs turn phenolic compounds, mainly hydroxycinnamic acids and catechins, into quinones, strong oxidants responsible for juice browning.
This reaction is very fast and occurs within 10-15 minutes beginning from when a grape berry is damaged or crushed.
SO2alternatives for minimizing juice oxidation
For enzymatic oxidation to happen, oxidation enzymes, O2 and the phenolic substrate must be present.
Consequently, the antioxidant strategy can operate on 3 fronts:
Reducing O2 solubilization in juice
Avoiding exposure to air, thus preventing the solubilization of oxygen in juice is the first step to prevent oxidation. In addition to using inert gas, chemical inertization is possible using compounds that can quickly react with oxygen before it can enter the enzymatic oxidation mechanism.
Contrary to common belief, SO2 is very slow in reacting with oxygen. Ascorbic acid is 170 times faster in reacting with oxygen than SO2. Its application (always in conjunction with SO2 and tannins to block oxygen peroxide produced by the oxidation of ascorbic acid), is an effective and cost-effective alternative to inert gas and allows for reduced SO2 additions.
Gallic and ellagic tannins also have a high capacity for O2 consumption and can be used to replace SO2.
Reducing oxidation enzyme activity
SO2 antioxidant effects depend on its capability of inhibiting juice oxidation enzymes. Up to 90% decrease tyrosinase activity has been observed upon the addition of 50 mg/L SO2, but higher dosages are necessary to effectively inhibit laccase, resulting in
negative consequences for alcoholic and malolactic fermentations and wine quality.
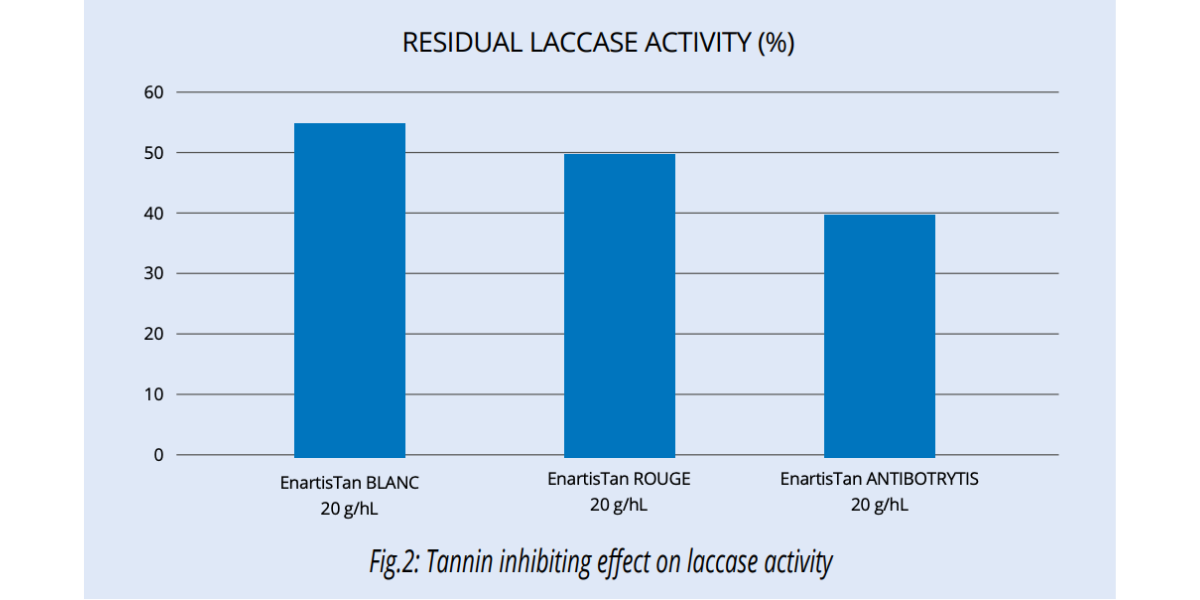
Enological tannins inhibit PPO activity and are more effective than SO2 in inactivating laccase (Fig. 2).
Recent trials have shown that the application of PVI/ PVP in white juice settling produces wines with a fresher color, higher content of aromatics and less sensitivity to oxidation. The reason is related to PVI/PVP’s ability to remove metals such as copper and iron.
During the juice stage, copper is a catalyzer of PPO activity and, similarly in wine, in combination with iron, it can catalyze the non-enzymatic oxidation of phenols.
Activated chitosan application during the juice stage is able to reduce laccase activity. The mechanism is not yet clear, chitosan could reduce laccase activity either by its capability in removing copper or by the direct reaction of positively charged chitosan with negatively charged laccase.
Finally, SO2 can reduce quinones produced by the enzymatic oxidation process back to a re-oxidizable form. This explains the SO2 bleaching effect when added to brown juice. Glutathione and cysteine have a similar ability, when added during the juice stage, they react with quinones formed from the oxidation of caftaric acid (the most abundant hydroxycinnamic acid in grape juice) by tyrosinase activity forming the “Grape Reaction Product” (GRP). GRP is colorless and is no longer a potential substrate for further oxidation by tyrosinase.
Removing phenolic compounds
Phenols are the main substrate of oxidation. Removing phenolic compounds with fining agents such as PVPP, animal or plant proteins and carbon is another way of minimizing the oxidation process.
ALTERNATIVES TO SO2 ANTIMICROBIAL ACTIVITY
The antimicrobial activity of sulfur dioxide is mainly due to the molecular form whose concentration depends on the free sulfur content, pH, temperature and alcohol content.
SO2 is effective in controlling both yeast and bacteria. It is able to interfere with the microbes metabolism in different ways: alteration of cell membrane permeability; alteration of enzymatic activity; inhibition of glycolysis; lipid modification; interaction with coenzymes and vitamins; destruction of thiamine; DNA and RNA transformation; and damage to structural proteins.
Every microorganism has a different sensitivity to SO2 therefore the quantity that is necessary to block the growth is specie dependent. Grape pH’s tendency to increase makes microbiological control with SO2 increasingly difficult.
Activated chitosan antimicrobial activity is much less dependent on juice pH and makes it a reliable alternative to SO2. It works by contact: the positive charges present on its surface attract negatively charged microorganisms in the juice. Subsequently, it
alters the permeability of the cell membrane and causes the microorganism to die from osmotic shock. Like SO2, chitosan is a non-specific antimicrobial compound and can control both yeast and bacteria.